FDA Grants Import Discretion of Bracco's Iodinated Contrast Medium Iomeron (iomeprol injection) to Address Supply Shortages
Bracco Diagnostics Inc. has announced that the U.S. Food and Drug Administration (FDA) granted import discretion of Iomeron (iomeprol injection) into the U.S. to address the ongoing iodinated contrast media shortage. The product addresses the need for the most advanced diagnostic imaging standards and will be temporarily available in the U.S. market starting at the end of August, 2022.
Contrast Media Imaging Technology News - 阿根廷vs乌拉圭直播

Arterion angiographic injection system

Bracco Acquires Contrast Injector Maker Swiss Medical Care
Ziehm Imaging Debuts Latest DR Suite

GE Healthcare Improves Digital Imaging Displays
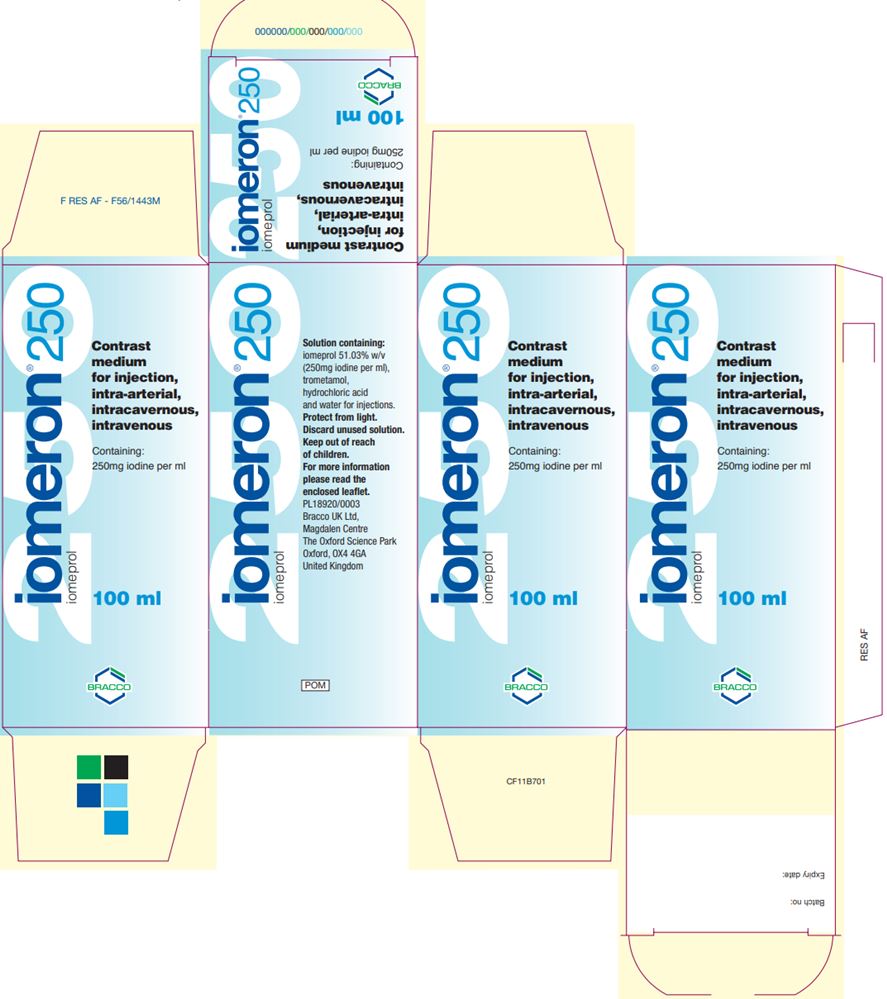
IOMERON- iomeprol injection injection, solution
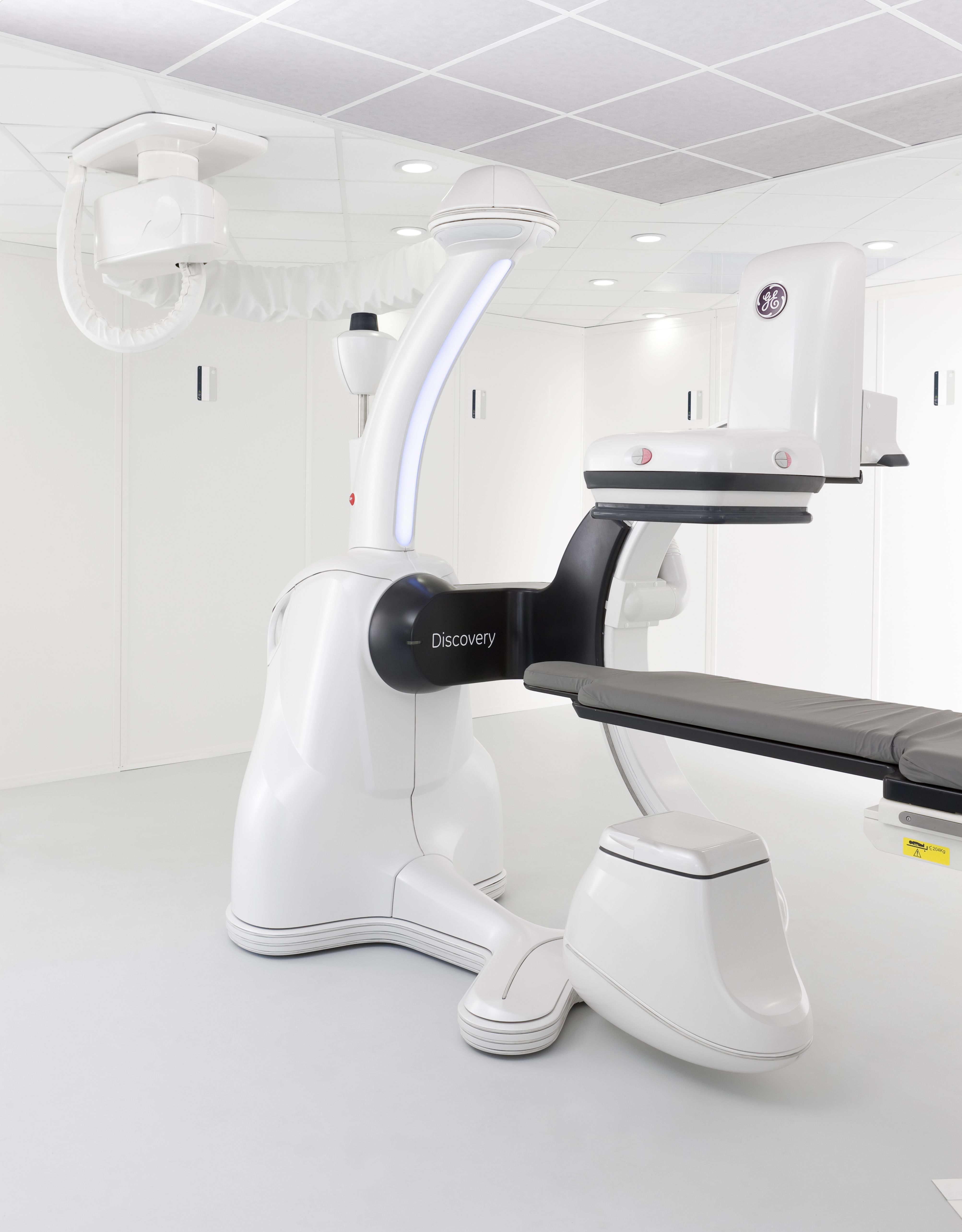
FDA Clears GE Healthcare's New Class of Angiography Systems
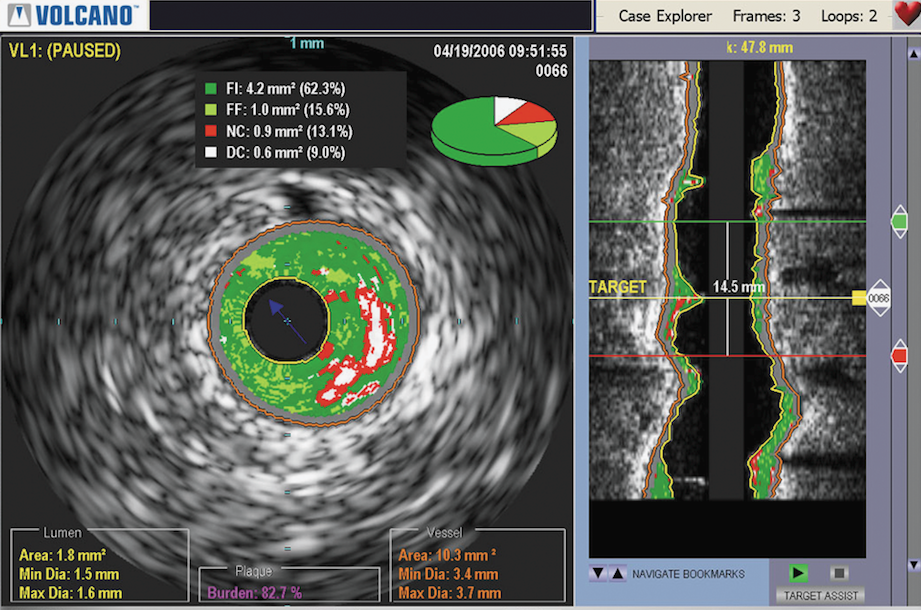
Philips Acquires Volcano for $1 Billion to Expand Position in Interventional Lab Market

image.cfm?name=UK-Iomeron-300-200ml-label.jpg&id=745554
articles • APPLIED RADIOLOGY